Wenn Du erschöpft bist, schleppst Du dich durch den Tag. Du brauchst aber Energie. Und genau die fehlt dir, wenn du Krebs hast oder gehabt hast.
Die Untire®-App hilft Brustkrebspatient:innen während und nach der Krebstherapie, ihre Energie zurückzugewinnen.
Energie für deinen Tag
Wir finden: Jede:r Fatigue-Patient:in ist eine:r zu viel. Mit einer zielgerichteten Therapie erhalten alle Betroffenen Hilfe. Die Untire-App macht‘s möglich.
Heute gehört mir
Maria (62): „Die Untire-App war sechs Monate lang meine beste Freundin. Es ist schön, jemanden an der Seite haben, der hin und wieder mal sagt: „Du machst das schon ganz richtig“ oder „Was du durchmachst, ist ganz normal.“
Offizielle DiGA
Untire-App ist die erste (vorläufig zugelassene) Digitale Gesundheitsanwendung (DiGA) bei krebsbedingter Erschöpfung
Sicher
Höchster Datenschutz – DSGVO- und DiGAV-konform
Kostenfrei
Die Kosten für Untire werden von allen gesetzlichen Krankenkassen übernommen
Mehr Energie
Mit der Untire-App lernst du Schritt für Schritt deinen eigenen Energiehaushalt besser kennen. Du erkennst immer schneller, was dir Energie verleiht und was deine Energiefresser sind. Diese Einsicht verhilft dir zu mehr Energie. Und du fühlst dich weniger erschöpft.
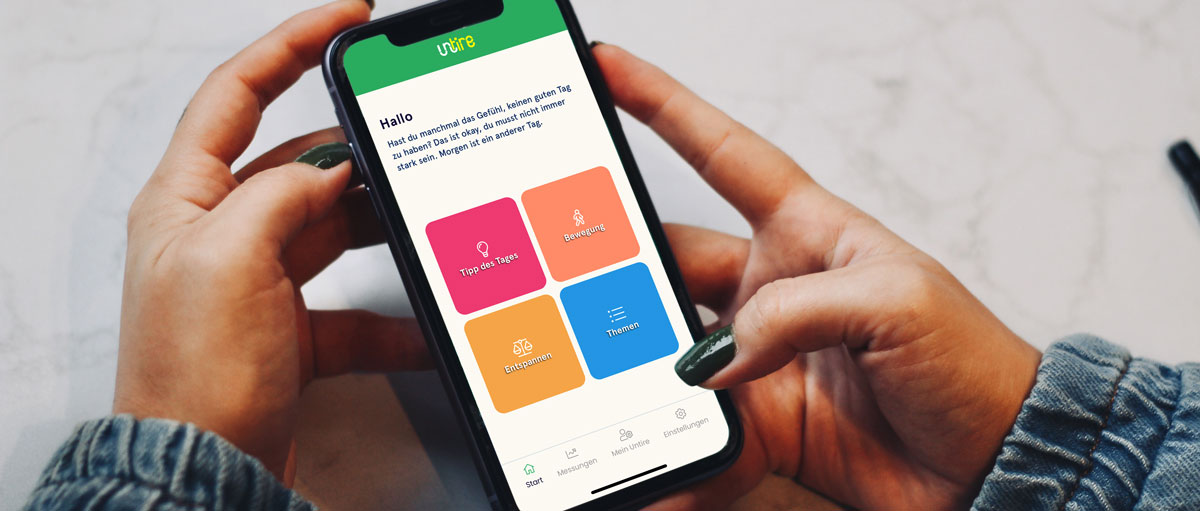
Einfach und praktisch
Deinen Energiehaushalt im Blick
Verschiedene Themenbereiche
Entspannungsübungen
Nutze die Untire-App
Die Untire-App wirkt am besten, wenn du sie dreimal wöchentlich mindestens 20 Minuten nutzt.
Videos, Informationen und täglichen Tipps

Austausch mit anderen Betroffenen in der Online Community